By C.A. Kotsanis, M.D.
Lyn Dart, R.D., L.D.
Christopher Harjes, M.S., CCC-A
Renay Miller, E.M.T.
DFW Ear, Nose, & Throat Associates Grapevine, Tx. (817) 481-6342
Study Profile
Autism is a developmental disorder of brain dysfunction with patients exhibiting a characteristic set of behaviors including impaired reciprocal social interaction impaired verbal and nonverbal communication, a marked restriction of activities and interests, and abnormal response to sensory stimuli. A global incidence of 4.5:10,000 children is reported, with a 4:1 ratio of males to females affected by autistic syndromes. There is increasing evidence that autism is etiologically a heterogeneous disorder involving neurobiological alteration in central nervous system functioning. Anatomical aberations of the cerebellum have been verified by MRI and confirmed by autopsy in autistic patients, and are presumed to initiate in utero. The basic pathology underlying autism has not yet been delineated.
Diagnosis usually occurs before the third year due to a display of aberrant behaviors, (1) evident from the first few weeks or months of life, or (2) evident after normal progressive development followed by a sudden loss of previously acquired behaviors (e.g. language). This rapid deterioration is typically reported to occur at approximately 12 to 24 months of age coupled with characteristics of the syndrome that may not have been evident earlier. Previous treatment of autism has primarily focused on pharmacological and/or behavioral therapy plus dietary and nutrition intervention. More recent applications involve allergy treatment and sound therapy (Auditory Enhancement training).
Research has identified, and studied in isolation, autistic subgroups with abnormal biologic markers associated with immunologic, nutritional, and audiologic mechanisms, but there exists a void in current literature without a clear definition of their individual role in the autistic syndrome. In addition, there have been no previous studies on the combined investigation and possible interrelationship between these factors.
Allergy
Comparative RAST and ALCAT analysis for foods, food dyes, molds, grasses, trees, weeds, epidermals and insects were evaluated. All positive results were verified by SET and multi-food tests. The general allergy profile revealed the following percentage of subjects with reactive levels:
1. Food dyes: 100% (ALCAT)
2. Foods: 67-100% (RAST – ALCAT – Skin)
3. Molds: 67-100% (RAST – ALCAT – Skin)
4. All other testing: 25% (RAST & Skin)
An immunocompetence screen including total serum IgE, IgG, IgA, IgM. CBC with differential, and CD antigens were also assessed. The following results were noted:
1. Non-IgE mediated (<25%)
2. IgG = 100% normal
3. IgM = 6% above normal
4. IgA = 17% below normal
5. Basophil: 75% above normal
6. Eosinophil :50% above normal
7. Platelets: 50% above normal
8. CD4+/CD8+/CD11+: 16-33% disordered profiles
9. CD2D+ = 100% above normal
Metabolic
Urinary analysis of neurochemical status was assessed for dopamine, norepinephrine, epinephrine, matanephrine, normetanaphrine, and serotonin. Disordered patterns were significant in 100% of subjects. Elevated levels of dopamine, epinephrine, and norepinephrine were more pronounced in 75-92%
To assess G.I. status in this group of children, the Comprehensive Digestive Stool Analysis (CDSA) and the Intestinal Permeability Assessment was completed on each subjects. Group results indicate the following dysfunctional profiles:
1. Impaired digestive capabilities: 92%
2. Abnormal microbial activity: 100%
-67% disordered bacteria
-67% elevated yeast
-50% parasites
3. GUT permeability: 75% leaky gut syndrome
This prospective study was initiated (1) to assess the biochemical and metabolic status, plus (2) to determine the efficacy of a multidisciplinary approach to treatment in a group of autistic children. The investigation will conclude a one year observation on September 30, 1993.
Subjects
Twelve autistic children, 11 boys and 1 girl, between the ages of 5-10 years, and satisfying the DSK-III-R criteria (American Psychiatric Association, 1987) for the diagnosis of autism, were selected to participate in this study. Pre-treatment substantiation of autistic characteristics were evaluated by parents using the Childhood Autism rating Scale (C.A.R.S.). The following primary behavioral criteria were noted for pre-, mid-, and post- treatment comparison:
1. Relating to people
2. Emotional response
3. Adaptation to change
4. Visual response
5. Verbal communication
6. Level and consistency of intellectual response
Medical Assessment
Physical Exam
An initial examination revealed normal weight and height for appropriate age index. The majority of children exhibited the typical allergic facies, allergic shiners, and clear thick post-nasal drainage. Also, most children showed overdeveloped primary and secondary sexual characteristics.
Due to the age, hyperkinetic condition, and lack of cooperation of the children, it was initially difficult to adequately examine and obtain blood samples for analysis. To secure patient safety, it was necessary to complete the examination and blood draw under general anesthesia. However, with time and additional experience, we were able to examine and draw blood in our office without sedation.
The office procedure involves 3 adults to safely immobilize the child on an examining table, leaving the physician free to examine the child and draw blood. Collection of blood from the antecubital fossa was secured with an 18-20 gauge intercath. To rule out the possible influence of general anesthesia on baseline blood analysis, we analysed blood from a group of non-anesthetized autistic children.
Nutrition
Dietary analysis included food frequency patterns, energy intake with percentage distribution of carbohydrates, protein, and fat, plus percentage of RDA’s for selected vitamins and minerals. Results show an average daily intake of 1699 kcal, with a range = 1317-2050 kcal (normal =2200 kcal). Percentage distribution of carbohydrates = 54.3% (231 gms), protein = 13.9% (59 gms), and fat = 31.8% (60 gms). Group food frequency patterns showing percentage of daily intake include:
1. Bread/cereals =26%
2. Beverages = 14% (carbonated & flavored fruit drinks)
3. Meat & poultry = 13.5% (chicken-breaded, prepared meats, beef-hamburgers)
4. Fruit = 13.5% (orange juice, apple juice, banana)
5. Snacks = 13.5% (chips, peanut butter, cookies/candy)
6. Combined = 9.5% (pizza, macaroni & cheese, spaghetti)
7. Dairy = 7% (cheese – yellow)
8. Vegetables = 3% (potato – fried)
Food dye reactivity was evident in 100% of the subjects: 67% orange, 58% red #2, 58% yellow #10, 50% red #3, 25% red #1, 8% green, 0% black.
Percentage of RDA’s for vitamins and minerals exhibit group patterns representative of decreased caloric intake coupled with infrequent nutrient dense food selections:
Decreased RDA intake (% subjects): Vitamin A = 50%
Vitamin D = 75 %
Vitamin E = 50%
Vita B6 = 33%
Calcium = 67%
Zinc = 100%
(Vitamin C, niacin, vitamin B12, phosphorus, magnesium, and iron were decreased in 8-25% of subjects. Thiamin, folic acid, and selenium were in excess of RDA for all subjects).
Bio-nutrient profiles measured intra and extra cellular response of circulating and functional vitamins, lymphocyte minerals, amino acids, and lymphocyte fatty acids. Initial inspection of group results revealed both the plasma amino patterns in 100% of the subjects. Although other profiles analyzed under sedation did not show the same pattern, we questioned the influence from general anesthesia (halothane). To investigate a possible relationship, the same profiles were evaluated in a group of four non-anesthetized autistic children. Results indicate similar patterns couples with distinct differences between groups. These findings belong in a separate report and will be addressed later. The following group patterns (% subjects) from baseline analysis were noted:
1. Decreased cellular response:
Vitamin A = 58%
Riboflavin (EGR) = 42%
Niacin (1-MMN) = 42%
Vitamin BG (EGOT) = 83%
(vitamin D, C, E, and thiamin (ETK) were decreased in 8-25% of subjects. Folic acid (FIGLU) and vitamin B12 (NMA) were within normal for all subjects.)
Comparative analysis between dietary intake and cellular response of same nutrients revealed inconsistent patterns within the group. The decreased levels of vitamin A, C, folic acid (FIGLU), and vitamin B12 (MMA) appear to reflect similarities between dietary intake and cellular response. In addition, the discrepency between dietary intake and cellular response of thiamin (ETK), riboflavin (EGR), niacin (1-NMN), and vitamin B6 (EGOT) may indicate a greater metabolic need and/or dysfunctional uptake.
2. The lymphocyte trace metals showed disordered cellular response of calcium (33%0, chromium (25%), magnesium (42%), manganese (50%), selenium (25%), and zinc (33%). Elevated and/or decreased levels of zinc, manganese, selenium, and chromium have been implicated in affecting inhibitory immune response, with suppression of antibody synthesis in vivo/vitro.
3. 24-hour urinary amino acids (collected prior to sedation) also revealed patterns to include decreased tyroine, carnosine, lysine, and hydroxylysine in all subjects. Consistently decreased levels have been implicated in suppressed uptake of amino acids due to incomplete or dysfunctional digestion in the gut. In addition, of interest to our subject profile, is the essential role tyrosine plays as a primary precursor to neurochemicals dopamine, norepinephrine and epinephrine.
4. Plasma amino acids revealed group patterns to include 100% of the subjects. Elevated levels of taurine, GABA, aspartic acid, and decreased arginine were eident in all subjects. This pattern may be representative of both autism and autism under general anesthesia. The elevated taurine and GABA were consistent with both anesthetized and non-anesthetized groups (though significantly different). Aspartic acid was not 100% elevated and arginine was not decreased in the non-anesthetized group. Arginine appears to play a direct role in immune functioning. This discrepency of elevated aspartic acid and/or decreased levels of arginine in the anesthetised group may indicate a immunodepressive role in general anesthesia.
5. The lymphocyte fatty acid profile showed 100% tendencies to elevated linoleic acid (essential), decreased arachidonic acid (essential), and decreased levels of mono-saturates oleic, nyristoleic, palmitoleic, and palmitelaldic acids. These essential fatty acids appear to play a key role in immune function, with deficit and/or excess concentrations affecting immunomodulation. Also, linoleic and arachidonic acid effect cell membrane fluidity, which in turn influence cell permeability and the behavior of membrane-associated enzymes and receptors which control intra and extra cellular functions. The regulation of cell membrane fluidity is currently under investigation as a possible mechanism for general anesthesia induction.
Audiology
An initial audiogram (play audiometry or traditional audiometry) for each subject was evaluated for behavioral and/or voluntary baseline and UCL thresholds at 125-8000 Hz based on normal developmental progression was also completed for each subject. Baseline evaluation revealed the following group profiles:
1. Hyperacusis = 75%
2. Deiayed speech = 100%
3. Non-verbal (babbling) = 25%
4. 2-3 words to sentence unclear = 50%
5. Conversational = 25%
GI intervention Treatment
1. Abnormal flora was treated specific for pathogen with regard to individual drug sensitivities. Length of treatment ranged from 5 days to 6 weeks depending on type of organism. The following medications/preparations were used:
– Antibacterials: Trimothoprin/sulfamethoxazole Amoxocillin
– Antifungals: Nystatin Citrus extract
– Antiparasitics: Metronidazole (giardia) Mabendazole (pin worms)
-Acidophilus: All patients were treated with lactobasilia mixtures for 12 months to re-establish flora
2. Pancreatic enzymes were recommended with main meals throughout the 12 months for subjects exhibiting impaired digestive functions.
3. All patients diagnosed with leaky gut syndrome received a preparation combining an amino acid (L- glutamine), fatty acid (gamma linoleic acid), complex carbohydrate (N-actyl-D-Glucosamina), antioxidant (gamma oryzanol) and vitamin E. Recommended dossage: one tab TID between meals for 2 months.
Auditory Enhancement Training (A.E.T.)
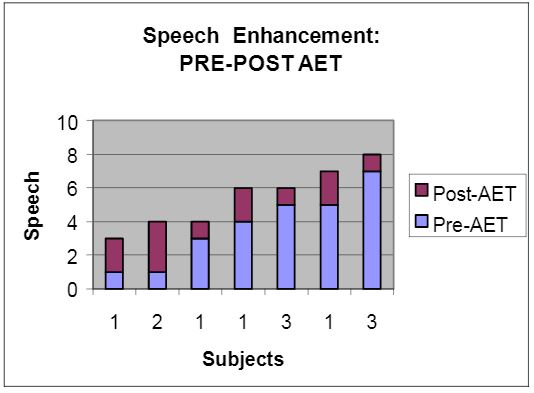
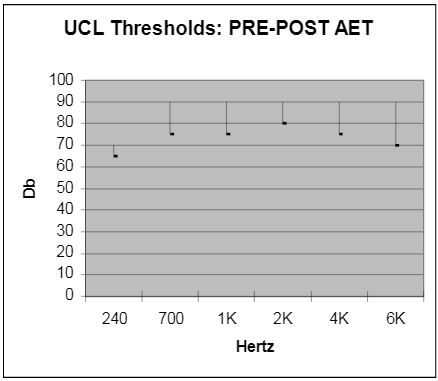
All children underwent play audiometry or traditional testing, pre-, mid-, and post-treatment. Each child participated in a ten day, 2 sessions per/day (1/2 hour per session with 4 hr/intervals between) training program. Pre-treatment UCL thresholds were abnormal in 75% of the children. Following Auditory Enhancement Training, UCL threshold levels were within normal range (see insert). In addition, a positive modification in speech enhancement was observed in 100% of the subjects post-A.E.T (see insert).
Allergy
1. Treat positive inhalants
2. Phenol free antigens (as needed)
3. Food dye elimination
4. Environmental clean-up
5. Filtered water
6. Food elimination & rotation diets
Nutrition
1. Dietary:
-Maximize nutrient intake
-Rotation & elimination diets
2. Bio-nutrient:-Therapeutic support included a comprehensive multi-vitamin/mineral preparation plus amino acid supplementation as needed.
Clinical Outcomes
This investigation has provided many curious and unexpected twists and turns in the ongoing inquiry of autism. In final viewing, it appears more questions have been raised versus answers given.
As clinicians, we initiated this study with 12 autistic children and subsequently embarked on a year long venture of uncertainty, excitement, and hope. In final viewing, the clinical outcomes proved of value to our patients.
Post-study assessment of treatment effectiveness showed positive responses in all 12 children. Parent evaluations coupled with clinical observations revealed group profiles exhibiting the following characteristics:
1. Increased:
Verbal and communicative skills
Attentive responses
Emotional responses
Logical application
Appropriate social behaviors
2. Decreased:
Hyperacusis
Impulsivity
Sensitivity to touch
3. Improved:
Eye contact
Memory skills
Ability to follow commands/instruction
Literature
Advanced Nutrition and Human Metabolism: Hunt SM, Groff JL. West Publishing Co., NY.; 1990
American Psychiatric Association, Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mortal Disorders, 3rd ed, revised. Washington DC: American Psychiatric Association; 1987.
Audio Tone Enhancer –Trainers Users Guide: BGC Enterprises, Inc., San Diego, CA; 1991
Autism – Nature, Diagnosis & Treatment: Dawson G (ed.). The Guilford Press, NY; 1989
Barbul A. Arginine and immune function. Nutr. (supp.) 1990; 6:53-58.
Bendich A. Vitamins and immunity. J Nutr. 1992; 122:601-603.
Besedovsky HO, Del Rey AE, Sorkin E. Immune-Neuroendocrine Interactions. J of Immuno. 1965;135:750-754.
Beyreiss J., Roth N, Beyer H. Coincidence of immune (atopic dermatitis) and behavioral (attention deficit) disorders in children: empirical data. Activ. Nerv. Sup. 1968;30:127-8.
Cao YZ, Maddox JF, Mastro AM. Selenium deficiency alters the lipoxygenase pathway and mitogenic response in bovine lymphocytes. J Nutr. 1952;122:2121-7
Cellular & Molecular Immunology: Abbas AK, Lichtman AH, Pober JS, W.B. Saunders Company, London, 1991.
Chandra RK, Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr.; 1991; 53:1087-1101.
Cheng S, Brunner EA. Inducing anesthesia with a GABE analog, THIP. Anesthesiology. 1985;63:147-151.
Children’s Nutrition: Lifshitz F, Finch NM, Lifshitz JZ. Department of Pediatrics, Cornell University Medical College, BY Jones & Bartlett Publishres, Boston; 1991.
Coleman M. The search for neurological subgroups in autism. Neurobiological Issues in Autism. Plenum Press, New York, NY 1987.
Coleman M. Nutritional treatments currently under investigation in autism. Clin Nutr. 1989; 8:210-212.
Fletcher MP, Gershwin ME, Keen CL, Hurley L. Trace element deficiencies and immune responsiveness in humans and animal models. Nutrition and immunology. Alan R. Lies, Inc., New York, NY, 1988.
Gershwin ME, Beach RS, Hurley LS –The potential impact of nutritional factors on immunological responsiveness. Nutrition and Immunity. Academic Press, Inc., New York, NY, 1985.
Handbook of Autism & Pervasive developmental Disorders: Cohen DJ, Donnellan AM, Paul R., John Wilery & Sons, 1987.
Hearing Equals behabior: Bernard G, Kaats Publishing Inc, New Canaan, CN, 1993 immunology: Kuby J, San Francisco State University. WH Freeman & Co, NY, 1992.
Kinsella JE Lookesh B, Broughton S, Whelan J. Dietary polyunsaturated fatty acids and elcosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition (supp). 1990, 6:24-44.
Klein A, Armstrong B. Hyperacusis & otitis media in individuals with Williams syndrome. J of Speech & Hearing Disorders. 1990; 55:339-344.
Leucocyte Typing IV –White Cell Differentiation Antigens: knapp W (ed.); Oxford University press, Oxford; 1989.
Launay JM, Burszteja C, Ferrari P, Catecholamines metabolism in infanmtile Autism: A controlled study of 22 Autistic children. J Autism & Devel Dis., 1987; 17:333-347
Membrane Interactions with General and Local Anesthetics: A review of Molecular Hypotheses of Anesthesia: Dluzewski AR, Halsey MJ, Simmonds AC. Pergamon Press Ltd, 1984.
Menage P, Thibault G, Martineau J, An IgE mechanism in autistic hypersensitivity? Biol Psychiatry, 1992;31:209-212.
Eaiten DJ, Massaro TF, Zucherman C. Vitamin and trace element assessment of autistic and learning disabled children. Nutr & behavior. 1984, 2:9-17
Raiten DJ, Massaro T. Perspectives on the nutritional ecology of autistic children. J Autism & Devel Dis. 1986;16:133-143.
Recommended Dietary Allowances: Food & Nutrition Board. National Research Council: 10th ed., National Academy Press; 1989.
Schreibman L. Autism. Newbury Park, CA: Sage Publications, Inc; 1988:2-78.
Shapiro AC, Wu D, Heydani Sn. Binosanoids derived from arachidonic and sicosapentaenoic acids inhibit T cell proliferative response. Prostaglandins. 1993;45:229-40.
Sherman AR. Zinc, cooper and iron nutritiure and immunity, J Nutr. 1992;122:604-609.
Singh VK, Warren RP, Odell JD. Changes of soluble interleukin-2, interleukin-2 receptor, TB antigen, and interleukin-1 in the serum of autistic children. Clin Immuno & Immunopathology. 1991; 61:440-455.
Slauson DO, Walker C, Kristensen F. Mechanisms of serotonin-Induced Lymphocyte Proliferation Inhibition. Cell Immuno; 1984; 23:112.21.
Jeda I, Hirakawa M, Arakawa K. Do Anesthetics Fluidize Membranes? Anesthesiology. 1986; 64:67-71.
Vernon J. Fathophysiology of Tinnitus: A special case –Hyperpousis & A proposed treatment. Am J of otology. 1987; 8:201-202.
Warren RP, Margaretten N, Pace NC. Immune abnormalities in patients with autism. J Autism & devel Dis.1986, 16:189-197.
Warren RP, Singh VK, Cole JD, Odell JD. Increased frequency of the null allele at the complement C4B locus in autism. Clin. Exp. Immunol. 1991;83:438-440.
Warren RP, Foster A, Margaretten N. Reduced Natural Killer Cell Activity in Autism. J Amer Acad Child Adol Psychol. 1987;26:333-335.
Allergy: please refer to traditional articles as recommended by the American Academy of Otolaryngic Allergy and the American Academy of Environmental Medicine.
Legend: Speech
1. Babbling
2. 1 word
3. 2-3 Words
4. Near Sentences
5. Sentences –Unclear
6. Sentences –Clear
7. Conversational
The Uncomfortable Loudness Thresholds (UCL) were obtained through soundfield. The lower bar of the arm was pre-AET and the upper arm was post-AET. Any frequency UCL below 90dBHL was labeled as hyperacusis. 75% of the subjects were labeled as hyperacusis pre-AET. Post-AET evaluations showed that 0% or none of the previous labeled subjects were hyperacusis. This was also correlated with parental questions on the Hearing Sensitivity Questionnaire pre-and-post-AET. This illustrates that the subjects were able to listen to louder or more intensified frequencies than before.