About the Author
Beth Ellen DiLuglio, MS, RDN, CCN, LDN is a Certified Clinical Nutritionist and Registered Dietitian with a 20 year history of certification in nutrition support. Beth earned a Master of Science degree in Human Nutrition from Columbia University in New York City. She earned her Bachelor’s in Biology/Psychology and developed a course of independent studies in Nutrition at Wheaton College in Norton, Massachusetts. Beth also completed “Applying Functional Medicine in Clinical Practice” through the Institute for Functional Medicine (IFM) and continues to train in the areas of Functional Laboratory Assessment and Functional Clinical Nutrition.
Introduction
Advances in genetic research confirm that we may be as different on the inside as we are on the outside. Genetic makeup is clearly reflected in outward appearance but genetic differences on the inside are not so obvious. Genes control production of the very elements that drive our biochemical machinery such as cofactors, enzymes, and other proteins. These elements are involved in metabolic activities from tissue building and repair to energy generation and detoxification. In turn genetic variations, particularly single nucleotide polymorphisms (SNPs), generate variations in enzyme activity, metabolic competence, and even nutrient requirements. SNPs and their expressions are believed to help explain susceptibility of certain individuals to specific diseases.[i]
SNPs significantly influence detoxification/biotransformation (ability to breakdown and eliminate endogenous hormones and exogenous toxins); individual response to certain drugs and medications; and even disease risk. Ongoing research reveals associations between SNPs and complex diseases including heart disease, diabetes, and cancer, and vascular disorders such as abdominal aortic aneurysm.[ii] [iii] [iv] [v] [vi] [vii] Additional factors such as consumption of alcohol may have a synergistic effect with certain SNPs, considerably increasing cancer risk, especially of the esophagus, stomach, and liver.[viii]
Background and rationale
Understanding SNPs and their consequences is essential to understanding both health and disease. SNPs that affect methylation (one-carbon transfer) have particularly wide-reaching effects. Adequate methylation is critical for DNA function and gene regulation; detoxification/biotransformation; homocysteine and amino acid metabolism; and glutathione synthesis. Altered methylation can ultimately affect DNA stability and gene expression.[ix] [x]
Folate plays a pivotal role in these reactions and hence is required by almost all tissues in the body including the brain.[xi] Therefore genetic variations in folate metabolism and one-carbon methylation reactions have a profound influence on disease risk. They are associated with elevated homocysteine levels, impaired methylation processes, and limited detoxification capacity. These factors can contribute to accelerated aging, chronic disease (e.g. cancer, cardiovascular disease, and neurodegenerative disorders), impaired gene regulation, poor drug clearance, and impaired neurotransmitter metabolism.
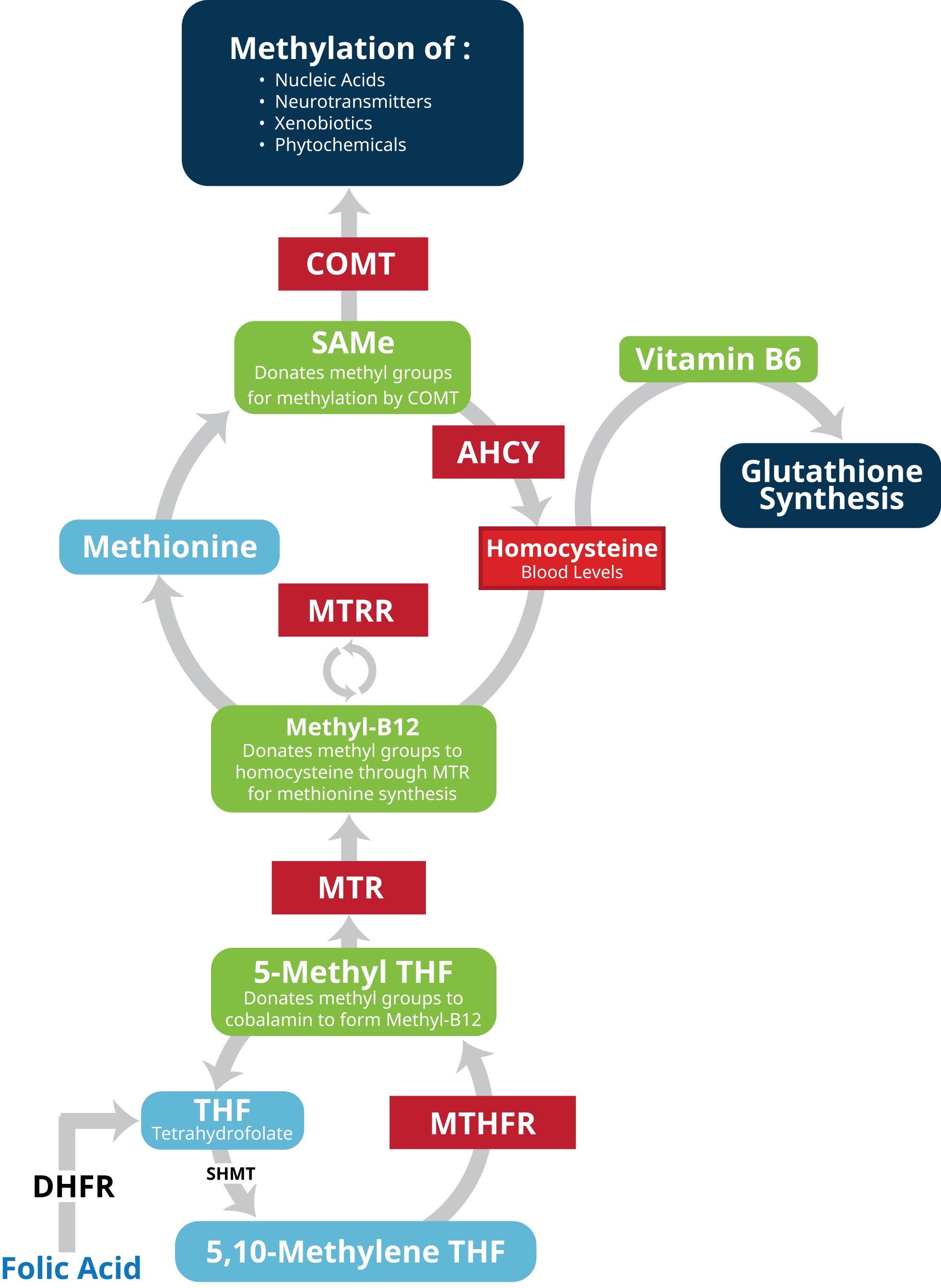
SNPs in the the methylenetetrahydrofolate reductase (MTHFR) gene and its role in methylation and homocysteine metabolism have been extensively researched. However, while knowing your patient’s MTHFR status is important it may not reflect the entire clinical picture. Research reveals that a number of genes, beyond MTHFR, influence not only methylation cycles but downstream byproducts and gene function.[xii] Such genes include 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), 5-methyltetrahydrofolate-homocysteine methyltransferase reductase (MTRR), catechol-O-methyltransferase (COMT), and AHCY (S-adenosylhomocysteine hydrolase). These genes play a major role in a patient’s methylation and detoxification capacity, neurotransmitter competence, and homocysteine metabolism.[xiii] [xiv] [xv] [xvi] [xvii] Measurement and monitoring of homocysteine levels are of equal importance as serum homocysteine can serve as a biomarker used to assess methylation.
Advanced Genetic Testing
Advanced genetic testing for SNPs allows a practitioner to take into account a patient’s unique genetic makeup when developing a care plan. Identifying SNPs and monitoring associated biomarkers lays the foundation for “personalized lifestyle medicine.” This focused approach enhances not only the practitioner’s ability to see the clinical picture clearly but enhances patient care and outcomes.[xviii] [xix] [xx] [xxi]
MTHFR testing strictly evaluates folate metabolism. Additional testing for SNPs in other genes that influence methylation potential and metabolic function provides a more comprehensive approach and may reflect a new set of “best practices.” Practitioners are acknowledging that testing for only MTHFR is no longer enough.
While knowing your patient’s MTHFR gene function is vital, it’s not enough to understand the whole clinical picture. MTR, MTRR, COMT, and AHCY are additional genes that play a major role in understanding patient methylation/detoxification capability. Knowing about the functioning of these genes can enable healthcare providers to address important chronic medical conditions. Methylation is also monitored using homocysteine levels. MTHFR only evaluates folic acid metabolism. Genetic variations in a variety of SNPs can influence methylation potential and this is why testing for only MTHFR is no longer enough.
Homocysteine
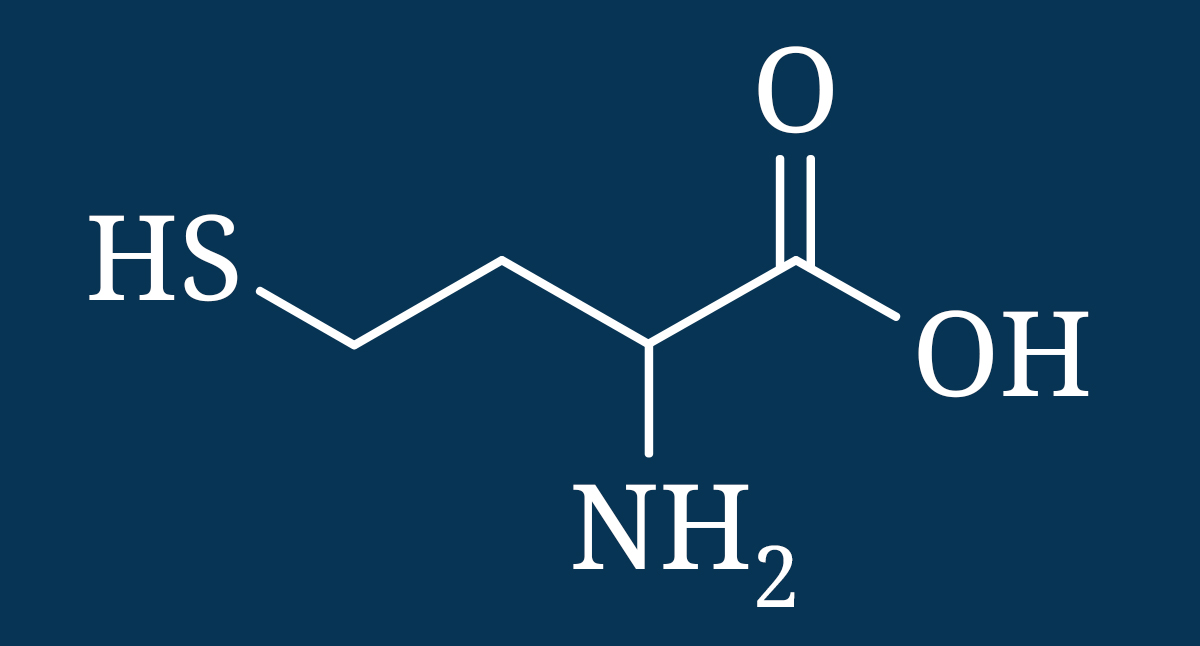
Homocysteine is an amino acid that is a part of methylation cycles yet at elevated levels it can become toxic to cells, damage blood vessels, promote oxidation, elicit an autoimmune response, increase risk of atherosclerosis, and contribute to dementia, depression, and neural tube defects.[xxii] [xxiii] [xxiv] [xxv] [xxvi] [xxvii] [xxviii] [xxix] [xxx] [xxxi] [xxxii] [xxxiii]
Though homocysteine is generated from methionine, it must be continuously recycled back to methionine (or on to cystathionine) in order to avoid accumulation. A number of genes play an active role in the overall processing of homocysteine and associated SNPs can disrupt that process and lead to toxic Hcy levels.[xxxiv] [xxxv] [xxxvi] [xxxvii] [xxxviii] [xxxix] [xl] Methionine/homocysteine balance is important for optimal health; the primary purpose of such balance is to ensure proper methylation by donating methyl groups for:
- DNA methylation (gene regulation)
- Regulation of neurotransmitters (e.g. epinephrine, norepinephrine, and dopamine)
- Detoxification of catecholamines from the environment
- Drug clearance (phase II liver detoxification)
- Homocysteine is also a precursor in the biosynthesis of L-cysteine for glutathione; glutathione is important for the detoxification of electrophilic compounds (metals).
Elevated serum homocysteine (Hcy) is a widely accepted marker for methionine/homocysteine imbalance, which is a genetically controlled process. Elevated homocysteine levels can lead to accelerated aging, cardiovascular disease and neurodegenerative disorders among others.[xli] [xlii] [xliii]
Single Nucleotide Polymorphisms
Methylenetetrahydrofolate reductase (MTHFR) is an enzyme that regulates folate metabolism in the body. Specifically MTHFR converts 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate (5-MTHF), the active form of folate. 5-MTHF then plays a key role in the single carbon transfer (i.e. methylation) reactions involved in the synthesis of nucleotides for DNA and RNA production; manufacture of S-adenosylmethionine (SAM-e); methylation of DNA, proteins, neurotransmitters, and phospholipids; and remethylation of homocysteine to methionine.[xlvi] [xlvii] MTHFR is especially critical in converting synthetic folic acid (which lacks coenzyme activity) to active 5-MTHF.[xlviii]
A SNP in the MTHFR gene (also known as methylenetetrahydrofolate reductase (NAD(P)H)) can ultimately jeopardize the methionine/homocysteine cycle leading to toxic levels of Hcy. Ultimately MTHFR SNPs can cause hyperhomocysteinemia (especially if folate levels are low); affect neurological, behavioral, and vascular health; contribute to birth defects, miscarriage, and preeclampsia; modulate cancer risk; and increase chronic disease risk.[xlix] [l] [li] [lii] [liii] [liv] [lv]
If a patient has acquired MTHFR SNPs from both parents (homozygous positive), she/he likely has significantly reduced MTHFR activity and a marked deficiency of 5-MTHF. If the patient received the SNP from only one parent (heterozygous positive) she/he may have suboptimal MTHFR function. At present the two most researched and understood MTHFR SNPs are 677C>T (C667T also called rs1801133) and 1298A>C (A1298C also called rs1801131). Testing these SNPs can help assess how well homocysteine can be cleared from the blood.[lvi] [lvii] [lviii] [lix] The final expression and influence of these variants can be affected by nutritional status, environment, genotype, and race/ethnicity.
Supplementation: When MTHFR SNPs are present, supplementation with 5-MTHF is recommended. Prolonged exposure to folate may be preferred to high doses that can be rapidly excreted.[lx] [lxi] Patient tolerance to supplementation should be monitored and nutrient administration individualized accordingly. It is equally important to promote intake of folate from food sources. Healthful folate sources include dark leafy greens, spinach, asparagus, legumes (e.g. lentils, chick peas, lima beans, black beans, kidney beans, pinto beans, green peas), Brussels sprouts, avocadoes, and broccoli.[lxii] [lxiii] [lxiv]
 MTR[lxv] [lxvi]
MTR[lxv] [lxvi]
5-methyltetrahydrofolate-homocysteine methyltransferase is the gene that codes for (i.e. provides instructions for) the methionine synthase enzyme (also known as cobalamin-dependent methionine synthase). Methionine synthase (MS) facilitates regeneration of methionine, an amino acid critical to protein synthesis in the body. During the process 5-MTHF serves as a methyl donor and vitamin B12 as the methyl transfer compound. In a first reaction, MS attaches the methyl group from 5-MTHF to cobalamin, forming a MS-methylcobalamin complex. The MS-methylcobalamin complex then transfers this methyl group to homocysteine, thus converting it into methionine.
During this process, cobalamin becomes oxidized over time and has to be reduced again to maintain proper function. During this step methionine synthase reductase (encoded by the MTRR gene) utilizes S-adenosylmethionine (SAM-e) as a methyl donor to regenerate methylcobalamin.[lxvii] Ultimately methionine synthase is crucial to amino acid metabolism; maintaining safe levels of homocysteine; and maintaining adequate cellular levels of methionine and SAM-e. SAM-e in turn is an essential methyl donor during DNA/RNA metabolism and neurotransmitter synthesis.
The MTR gene mutations C3518T and A2756G affect function of the enzyme even in the heterozygous form. They are associated with higher homocysteine levels and may lead to hypomethylation.
Supplementation: MTR SNPs may manifest as a methylcobalamin deficiency.[lxviii] When genetic mutations are present in the MTR gene, supplementation with methylcobalamin (methylated form of vitamin B12) is recommended.
 MTRR[lxix] [lxx]
MTRR[lxix] [lxx]
The 5-methyltetrahydrofolate-homocysteine methyltransferase reductase gene codes for methionine synthase reductase (MSR), an enzyme that remethylates cobalamin to methylcobalamin and reduces or “reactivates” oxidized methionine synthase so that conversion of homocysteine to methionine can continue.
SNPs in the MTRR gene may lead to an MSR enzyme that is less effective or ineffective, contributing to megaloblastic anemia as well as elevated levels of homocysteine in blood and urine. A common MTRR SNP known as A66G appears to be specifically associated with neural tube defects, colorectal cancer, cardiovascular disease, and increased risk of Down syndrome.[lxxi] [lxxii] [lxxiii] [lxxiv] The A66G polymorphism. In combination with the MTHFR C677T polymorphism, MTRR genotypes AG/GG influence total plasma homocysteine levels. Additionally, the combination of the genetic polymorphisms in MTRR and MTHFR was linked to an increase in DNA damage as measured by micronucleus frequency (MN).
Supplementation: When genetic mutations are present in the MTRR gene, supplementation with SAM-e is recommended.
 COMT[lxxv] [lxxvi]
COMT[lxxv] [lxxvi]
The catechol-O-methyltransferase (COMT) gene codes for an enzyme of the same name. The enzyme itself is produced in two forms. The long form, membrane-bound catechol-O-methyltransferase (MB-COMT), is synthesized primarily by nerve cells in the brain. Here the COMT enzyme facilitates degradation of catecholamine neurotransmitters (e.g. dopamine, epinephrine, and norepinephrine) by catalyzing the transfer of a methyl group from SAM-e to the catecholamines. The same activity facilitates the breakdown of catechol drugs (e.g. those used in treatment of hypertension, asthma, and Parkinson’s disease). COMT plays an especially important role in maintaining neurotransmitter balance in the prefrontal cortex where personality, behavior, emotion, abstract thinking, and short-term memory are managed.[lxxvii] The shorter form of the COMT enzyme (soluble catechol-O-methyltransferase (S-COMT) helps control hormone levels the blood, liver, kidneys, and other tissues.
Two SNPs, Val108/158Met and Ala52/102Thr, are known to alter COMT methylation capacity.[lxxviii] These two SNPs of the COMT gene are associated in literature with[lxxix] [lxxx] [lxxxi] [lxxxii] [lxxxiii] [lxxxiv] [lxxxv]
- Impaired DNA methylation
- Impaired neurotransmitter metabolism
- Decreased drug metabolism (important in neurodegenerative disorders)
- Decreased detoxification of toxic catecholamines from the environment
- Chronic widespread pain and fibromyalgia
- Involvement in the manifestation of a variety of human disorders, including estrogen-induced cancers, Parkinson’s disease, depression, and hypertension
Supplementation: When genetic mutations are present, supplementation with SAMe is often recommended. Attention should be paid to other medications being taken, including anti-depressants.
 AHCY[lxxxvi] [lxxxvii] [lxxxviii]
AHCY[lxxxvi] [lxxxvii] [lxxxviii]
The adenosylhomocysteinase (AHCY) gene codes for S-adenosylhomocysteine hydrolase, the enzyme that converts S-adenosylhomocysteine (AdoHcy) to adenosine and homocysteine in a reversible hydrolysis reaction that occurs during methioinine metabolism.[lxxxix] This conversion is an integral step in methylation and its related functions including the carrying out of DNA instructions, regulation of protein and lipid metabolism, and neurotransmitter processing.
Initially SAM-e (also known as AdoMet) is converted to AdoHcy after donating its methyl group in a step catalyzed by COMT (see COMT). AdoHcy is then converted by S-adenosylhomocysteine hydrolase back to homocysteine. (Nicotinamide adenine dinucleotide (NADH) serves in this reaction as a co-factor for AHCY.) The ratio or balance between SAM-e and AdoHcy is referred to as methylation potential. Because AdoHcy inhibits methylation, it is crucial that conversion of AdoHcy to homocysteine occur immediately in order to maintain optimal methylation potential.
Several genetic polymorphisms (SNPs) in AHCY are known to alter activity and expression of the S-adenosylhomocysteine hydrolase enzyme, leading to elevated AdoHcy concentrations and impaired methylation potential. Recent studies show association between those mutations and poor methylation potential, severe myopathies, developmental delays, and hypermethioninemia.
Supplementation: When genetic mutations are present, supplementation with NADH (nicotinamide adenine dinucleotide + hydrogen) is often recommended. Supplementation with SAMe may also be recommended.
Summary
There is a growing body of knowledge and awareness with regard to nutrigenomics, nutrigenetics, and functional genetic testing. This research supports the premise in functional medicine that “genes load the gun, but environment pulls the trigger.” The recognition that genes, nutrients, and the environment interact on such a fundamental level is an important component of comprehensive healthcare. In view of this knowledge, hopefully testing for SNPs will become routine and correcting for these variants will become best practice.[xc]
Literature
[i] Liu O, Li JR, Gong M, Xu M, Du J, Zhang HJ. Genetic analysis of six SNPs in candidate genes associated with high cross-race risk of development of thoracic aortic aneurysms and dissections in Chinese Han population. Acta Pharmacol Sin. 2010 Oct;31(10):1376-80. doi: 10.1038/aps.2010.159. Epub 2010 Sep 27. PubMed PMID: 20871623; PubMed Central PMCID: PMC4012906.
[ii] Liu O, Li JR, Gong M, Xu M, Du J, Zhang HJ. Genetic analysis of six SNPs in candidate genes associated with high cross-race risk of development of thoracic aortic aneurysms and dissections in Chinese Han population. Acta Pharmacol Sin. 2010 Oct;31(10):1376-80. doi: 10.1038/aps.2010.159. Epub 2010 Sep 27. PubMed PMID: 20871623; PubMed Central PMCID: PMC4012906.
[iii] NIH. Genetics Home Reference. What are single nucleotide polymorphisms (SNPs)? Retrieved March 9, 2016 from https://ghr.nlm.nih.gov/handbook/genomicresearch/snp
[iv] Chang SC, Chang PY, Butler B, Goldstein BY, Mu L, Cai L, You NC, Baecker A, Yu SZ, Heber D, Lu QY, Li L, Greenland S, Zhang ZF. Single nucleotide polymorphisms of one-carbon metabolism and cancers of the esophagus, stomach, and liver in a Chinese population. PLoS One. 2014 Oct 22;9(10):e109235. doi:10.1371/journal.pone.0109235. eCollection 2014. PubMed PMID: 25337902; PubMed Central PMCID: PMC4206280.
[v] Figueiredo JC, Grau MV, Wallace K, Levine AJ, Shen L, Hamdan R, Chen X, Bresalier RS, McKeown-Eyssen G, Haile RW, Baron JA, Issa JP. Global DNA hypomethylation (LINE-1) in the normal colon and lifestyle characteristics and dietary and genetic factors. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1041-9. doi: 10.1158/1055-9965.EPI-08-0926. Epub 2009 Mar 31. PubMed PMID: 19336559; PubMed Central PMCID: PMC2712652.
[vi] Gaughan DJ, Kluijtmans LA, Barbaux S, McMaster D, Young IS, Yarnell JW, Evans A, Whitehead AS. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001 Aug;157(2):451-6. PubMed PMID: 11472746.
[vii] Giusti B, Saracini C, Bolli P, Magi A, Sestini I, Sticchi E, Pratesi G, Pulli R, Pratesi C, Abbate R. Genetic analysis of 56 polymorphisms in 17 genes involved in methionine metabolism in patients with abdominal aortic aneurysm. J Med Genet. 2008 Nov;45(11):721-30. doi: 10.1136/jmg.2008.057851. Epub 2008 Jul 17. PubMed PMID: 18635682.
[viii] Chang SC, Chang PY, Butler B, Goldstein BY, Mu L, Cai L, You NC, Baecker A, Yu SZ, Heber D, Lu QY, Li L, Greenland S, Zhang ZF. Single nucleotide polymorphisms of one-carbon metabolism and cancers of the esophagus, stomach, and liver in a Chinese population. PLoS One. 2014 Oct 22;9(10):e109235. doi:10.1371/journal.pone.0109235. eCollection 2014. PubMed PMID: 25337902; PubMed Central PMCID: PMC4206280.
[ix] Giusti B, Saracini C, Bolli P, Magi A, Sestini I, Sticchi E, Pratesi G, Pulli R, Pratesi C, Abbate R. Genetic analysis of 56 polymorphisms in 17 genes involved in methionine metabolism in patients with abdominal aortic aneurysm. J Med Genet. 2008 Nov;45(11):721-30. doi: 10.1136/jmg.2008.057851. Epub 2008 Jul 17. PubMed PMID: 18635682.
[x] Figueiredo JC, Grau MV, Wallace K, Levine AJ, Shen L, Hamdan R, Chen X, Bresalier RS, McKeown-Eyssen G, Haile RW, Baron JA, Issa JP. Global DNA hypomethylation (LINE-1) in the normal colon and lifestyle characteristics and dietary and genetic factors. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1041-9. doi: 10.1158/1055-9965.EPI-08-0926. Epub 2009 Mar 31. PubMed PMID: 19336559; PubMed Central PMCID: PMC2712652.
[xi] Araújo JR, Martel F, Borges N, Araújo JM, Keating E. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res Rev. 2015 Jul;22:9-19. doi: 10.1016/j.arr.2015.04.005. Epub 2015 May 2. Review. PubMed PMID: 25939915.
[xii] Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004 Mar 1;159(5):423-43. Review. PubMed PMID: 14977639. Retrieved March 12, 2016 from http://aje.oxfordjournals.org/content/159/5/423.long
[xiii] U.S. National Library of Medicine. MTHFR. Retrieved March 10, 2016 from
https://ghr.nlm.nih.gov/gene/MTHFR
[xiv] Barbosa PR, Stabler SP, Machado AL, Braga RC, Hirata RD, Hirata MH, Sampaio-Neto LF, Allen RH, Guerra-Shinohara EM. Association between decreased vitamin levels and MTHFR, MTR and MTRR gene polymorphisms as determinants for elevated total homocysteine concentrations in pregnant women. Eur J Clin Nutr. 2008 Aug;62(8):1010-21. Epub 2007 May 23. PubMed PMID: 17522601.
[xv] NIH. MTR. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/MTR
[xvi] NIH. MTRR. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/MTRR
[xvii] NIH. COMT. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/COMT
[xviii] Schwartz B. New criteria for supplementation of selected micronutrients in the era of nutrigenetics and nutrigenomics. Int J Food Sci Nutr. 2014 Aug;65(5):529-38. doi: 10.3109/09637486.2014.898258. Epub 2014 Mar 13. Review. PubMed PMID: 24625102. Retrieved March 10, 2016 from https://www.researchgate.net/profile/Betty_Schwartz2/publication/260808417_New_criteria_for_supplementation_of_selected_micronutrients_in_the_era_of_nutrigenetics_and_nutrigenomics/links/552a163c0cf29b22c9bf6904.pdf
[xix] Minich DM, Bland JS. Personalized lifestyle medicine: relevance for nutrition
and lifestyle recommendations. ScientificWorld Journal. 2013 Jun 26;2013:129841.
doi: 10.1155/2013/129841. Print 2013. PubMed PMID: 23878520; PubMed Central
PMCID: PMC3710624. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3710624/
[xx] Stover PJ. Influence of human genetic variation on nutritional requirements.
Am J Clin Nutr. 2006 Feb;83(2):436S-442S. Review. PubMed PMID: 16470009. Retrieved March 10, 2016 from http://ajcn.nutrition.org/content/83/2/436S.long
[xxi] Subbiah MT. Nutrigenetics and nutraceuticals: the next wave riding on
personalized medicine. Transl Res. 2007 Feb;149(2):55-61. Review. PubMed PMID:
17240315.
[xxii] Per?a-Kaján J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32(4):561-72. Epub 2007 Feb 7. Review. PubMed PMID:
17285228.
[xxiii] Perna AF, Ingrosso D, Lombardi C, Acanfora F, Satta E, Cesare CM, Violetti E, Romano MM, De Santo NG. Possible mechanisms of homocysteine toxicity. Kidney Int Suppl. 2003 May;(84):S137-40. Review. PubMed PMID: 12694330.
[xxiv] Reif A, Pfuhlmann B, Lesch KP. Homocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2005 Sep;29(7):1162-8. PubMed PMID: 16055253.
[xxv] Schwartz B. New criteria for supplementation of selected micronutrients in the era of nutrigenetics and nutrigenomics. Int J Food Sci Nutr. 2014 Aug;65(5):529-38. doi: 10.3109/09637486.2014.898258. Epub 2014 Mar 13. Review. PubMed PMID: 24625102. Retrieved March 10, 2016 from https://www.researchgate.net/profile/Betty_Schwartz2/publication/260808417_New_criteria_for_supplementation_of_selected_micronutrients_in_the_era_of_nutrigenetics_and_nutrigenomics/links/552a163c0cf29b22c9bf6904.pdf
[xxvi] Smith, A. D., & Refsum, H. (2016). Homocysteine, B Vitamins, and Cognitive Impairment. Annual Review of Nutrition, 36(1). Retrieved March 14, 2016 from http://www.annualreviews.org/doi/abs/10.1146/annurev-nutr-071715-050947
[xxvii] Ji Y, Tan S, Xu Y, Chandra A, Shi C, Song B, Qin J, Gao Y. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: a meta-analysis. Neurology. 2013 Oct 8;81(15):1298-307. doi: 10.1212/WNL.0b013e3182a823cc. Epub 2013 Sep 18. PubMed PMID: 24049135.
[xxviii] Gaughan DJ, Kluijtmans LA, Barbaux S, McMaster D, Young IS, Yarnell JW, Evans A, Whitehead AS. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001 Aug;157(2):451-6. PubMed PMID: 11472746.
[xxix] Miller AL. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev. 2003 Feb;8(1):7-19. Review. PubMed PMID: 12611557. Retrieved March 10, 2016 from http://www.altmedrev.com/publications/8/1/7.pdf
[xxx] Sharma M, Tiwari M, Tiwari RK. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin Pharmacol Toxicol. 2015 Nov;117(5):287-96. doi: 10.1111/bcpt.12424. Epub 2015 Jun 19. Review. PubMed PMID: 26036286.
[xxxi] Kamat PK, Vacek JC, Kalani A, Tyagi N. Homocysteine Induced Cerebrovascular Dysfunction: A Link to Alzheimer’s Disease Etiology. Open Neurol J. 2015 Jun 24;9:9-14. doi: 10.2174/1874205X01509010009. eCollection 2015. PubMed PMID: 26157520; PubMed Central PMCID: PMC4485324.
[xxxii] Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014 Oct;10(4):281-8. doi: 10.3988/jcn.2014.10.4.281. Epub 2014 Oct 6. Review. Erratum in: J Clin Neurol. 2015 Jan;11(1):106. PubMed PMID: 25324876; PubMed Central PMCID: PMC4198708.
[xxxiii] Lutz UC. Alterations in homocysteine metabolism among alcohol dependent patients–clinical, pathobiochemical and genetic aspects. Curr Drug Abuse Rev. 2008 Jan;1(1):47-55. Review. PubMed PMID: 19630705.
[xxxiv] Sharp L, Little J. Polymorphisms in Genes Involved in Folate Metabolism and Colorectal Neoplasia: AHuGE Review. Am. J. Epidemiol. (2004) 159(5):423–443.
[xxxv] Figueiredo JC, Grau MV, Wallace K, Levine AJ, Shen L, Hamdan R, Chen X, Bresalier RS, McKeown-Eyssen G, Haile RW, Baron JA, Issa JP. Global DNA Hypomethylation (LINE-1) in the Normal Colon and Lifestyle Characteristics, Dietary and Genetic Factors. Cancer Epidemiol Biomarkers Prev. 2009 April; 18(4): 1041-1049
[xxxvi] Watkins D, Rosenblatt DS. Update and new concepts in vitamin responsive disorders of folate transport and metabolism J Inherit Metab Dis. 2012 Jul;35(4):665-70.
[xxxvii] Barbosa PR, Stabler SP, Machado AL, Braga RC, Hirata RD, Hirata MH, Sampaio-Neto LF, Allen RH, Guerra-Shinohara EM. Association between decreased vitamin levels and MTHFR, MTR and MTRR gene polymorphisms as determinants for elevated total homocysteine concentrations in pregnant women. Eur J Clin Nutr. 2008 Aug;62(8):1010-21. Epub 2007 May 23. PubMed PMID: 17522601.
[xxxviii] Dean L. Methylenetetrahydrofolate Reductase Deficiency. 2012 Mar 8. In: Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Retrieved March 9, 2016 from: http://www.ncbi.nlm.nih.gov/books/NBK66131/
[xxxix] Giusti B, Sestini I, Saracini C, Sticchi E, Bolli P, Magi A, Gori AM, Marcucci
R, Gensini GF, Abbate R. High-throughput multiplex single-nucleotide polymorphism
(SNP) analysis in genes involved in methionine metabolism. Biochem Genet. 2008
Aug;46(7-8):406-23. doi: 10.1007/s10528-008-9159-5. Epub 2008 Apr 22. PubMed
PMID: 18427977.
[xl] Santilli F, Davì G, Patrono C. Homocysteine, methylenetetrahydrofolate reductase, folate status and atherothrombosis: A mechanistic and clinical perspective. Vascul Pharmacol. 2016 Mar;78:1-9. doi: 10.1016/j.vph.2015.06.009. Epub 2015 Jun 22. Review. PubMed PMID: 26111718.
[xli] Cavalieri EL, Li KM, Balu N, Saeed M, Devanesan P, Higginbotham S, Zhao J, Gross ML, Rogan EG.. Catechol ortho-quinones: the electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis. 2002 Jun;23(6):1071-7.
21 Yager JD. Catechol-O-methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug Discov Today Dis Mech. 2012 June 1; 9(1-2).
[xlii] Yager JD. Catechol-O-methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug Discov Today Dis Mech. 2012 June 1; 9(1-2).
[xliii] Di Renzo L, Marsella LT, Sarlo F, Soldati L, Gratteri S, Abenavoli L, De Lorenzo A. C677T gene polymorphism of MTHFR and metabolic syndrome: response to dietary intervention. J Transl Med. 2014 Nov 29;12(1):329.
[xliv] U.S. National Library of Medicine. MTHFR. Retrieved March 10, 2016 from
https://ghr.nlm.nih.gov/gene/MTHFR
[xlv] National Center for Biotechnology Information. MTHFR. Retrieved March 10, 2016 from
http://www.ncbi.nlm.nih.gov/gene/4524
[xlvi] Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000 May 1;151(9):862-77. Review. PMID: 10791559.
[xlvii] Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014 Jan 1;533(1):11-20. doi: 10.1016/j.gene.2013.09.063. Epub 2013 Oct 1. Review. PubMed PMID: 24091066.
[xlviii] Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate:
comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet.
2010 Aug;49(8):535-48. doi: 10.2165/11532990-000000000-00000. Review. PubMed
PMID: 20608755.
[xlix] Reilly R, McNulty H, Pentieva K, et al. MTHFR 677TT genotype and disease risk: is there a modulating role for B-vitamins? Proc Nutr Soc. 2014 Feb;73(1):47-56. doi: 10.1017/S0029665113003613. PMID: 24131523.
[l] Neggers YH. Increasing prevalence, changes in diagnostic criteria, and nutritional risk factors for autism spectrum disorders. ISRN Nutr. 2014 Feb 13;2014:514026. doi: 10.1155/2014/514026. eCollection 2014. Review. PMID: 24967269
[li] Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014 Jan 1;533(1):11-20. doi: 10.1016/j.gene.2013.09.063. Review. PMID: 24091066.
[lii] Schwahn B, Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences. Am J Pharmacogenomics. 2001;1(3):189-201. Review. PMID: 12083967.
[liii] Sánchez-Marín B, Grasa JM. [Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in ischemic vascular disease]. Rev Neurol. 2006 Nov 16-30;43(10):630-6. Review. Spanish. PMID: 17099857.
[liv] Dean L. Methylenetetrahydrofolate Reductase Deficiency. 2012 Mar 8. In: Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Retrieved March 9, 2016 from: http://www.ncbi.nlm.nih.gov/books/NBK66131/
[lv] Dean L. Methylenetetrahydrofolate Reductase Deficiency. 2012 Mar 8. In: Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Retrieved March 9, 2016 from: http://www.ncbi.nlm.nih.gov/books/NBK66131/
[lvi] Toffoli G, De Mattia E. Pharmacogenetic relevance of MTHFR polymorphisms. Pharmacogenomics. 2008 Sep;9(9):1195-206. doi: 10.2217/14622416.9.9.1195. Review. PMID: 18781847.
[lvii] West AA, Caudill MA. Genetic variation: impact on folate (and choline)
bioefficacy. Int J Vitam Nutr Res. 2010 Oct;80(4-5):319-29. doi:
10.1024/0300-9831/a000040. Review. PMID: 21462116.
[lviii] Schwartz B. New criteria for supplementation of selected micronutrients in the era of nutrigenetics and nutrigenomics. Int J Food Sci Nutr. 2014 Aug;65(5):529-38. doi: 10.3109/09637486.2014.898258. Epub 2014 Mar 13. Review. PubMed PMID: 24625102. Retrieved March 10, 2016 from https://www.researchgate.net/profile/Betty_Schwartz2/publication/260808417_New_criteria_for_supplementation_of_selected_micronutrients_in_the_era_of_nutrigenetics_and_nutrigenomics/links/552a163c0cf29b22c9bf6904.pdf
[lix] Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988 Oct;43(4):414-21. PubMed PMID: 3177384; PubMed Central PMCID: PMC1715503.
[lx] Duncan TM, Reed MC, Nijhout HF. The relationship between intracellular and plasma levels of folate and metabolites in the methionine cycle: a model. Mol Nutr Food Res. 2013 Apr;57(4):628-36. doi: 10.1002/mnfr.201200125. Epub 2012 Nov 12. PubMed PMID: 23143835; PubMed Central PMCID: PMC3786706.
[lxi] Venn BJ, Green TJ, Moser R, Mann JI. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo-controlled study. Am J Clin Nutr. 2003 Mar;77(3):658-62. PubMed PMID: 12600857.
[lxii] Linus Pauling Institute Micronutrient Information Center. Folate. Updated June 2014. Retrieved March 10, 2016 from http://lpi.oregonstate.edu/infocenter/vitamins/fa/
[lxiii] National Institutes of Health Office of Dietary Supplements. Folate. Updated February 11, 2016. Retrieved March 10, 2016 from http://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
[lxiv] USDA National Nutrient Database Release 27. Retrieved March 10, 2016 from http://ndb.nal.usda.gov/ndb/nutrients/report?nutrient1=432&nutrient2=&nutrient3=&fg=&max=25&subset=0&offset=0&sort=c&totCount=6714&measureby=m
[lxv] NIH. MTR. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/MTR
[lxvi] National Center for Biotechnology Information. MTR. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/gene/4548
[lxvii] Watkins D, Ru M, Hwang HY, Kim CD, Murray A, Philip NS, Kim W, Legakis H, Wai T, Hilton JF, Ge B, Doré C, Hosack A, Wilson A, Gravel RA, Shane B, Hudson TJ, Rosenblatt DS. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am J Hum Genet. 2002 Jul;71(1):143-53. Epub 2002 May 30. PubMed PMID: 12068375; PubMed Central PMCID: PMC384971. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC384971/
[lxviii] Giusti B, Saracini C, Bolli P, Magi A, Sestini I, Sticchi E, Pratesi G, Pulli R, Pratesi C, Abbate R. Genetic analysis of 56 polymorphisms in 17 genes involved in methionine metabolism in patients with abdominal aortic aneurysm. J Med Genet. 2008 Nov;45(11):721-30. doi: 10.1136/jmg.2008.057851. Epub 2008 Jul 17. PubMed PMID: 18635682.
[lxix] NIH. MTRR. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/MTRR
[lxx] National Center for Biotechnology Information. MTRR. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/gene/4552
[lxxi] Wu PP, Tang RN, An L. A meta-analysis of MTRR A66G polymorphism and colorectal cancer susceptibility. J BUON. 2015 May-Jun;20(3):918-22. Review. PubMed PMID: 26214647.
[lxxii] NIH. MTRR. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/MTRR
[lxxiii] Gaughan DJ, Kluijtmans LA, Barbaux S, McMaster D, Young IS, Yarnell JW, Evans A, Whitehead AS. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001 Aug;157(2):451-6. PubMed PMID: 11472746.
[lxxiv] Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, Pogribna M, Rozen R, James SJ. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Genet. 2000 Sep;67(3):623-30. Epub 2000 Aug 7. PubMed PMID: 10930360; PubMed Central PMCID: PMC1287522.
[lxxv] NIH. COMT. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/COMT
[lxxvi] National Center for Biotechnology Information. COMT. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/gene/1312
[lxxvii] NIH. COMT. Retrieved March 10, 2016 from https://ghr.nlm.nih.gov/gene/COMT
[lxxviii] Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the New Selective COMT Inhibitors. Pharmacological Reviews December 1, 1999 vol. 51 no. 4 593-628.
[lxxix] Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-Methyltransferase (COMT)-mediated Metabolism of Catechol Estrogens Comparison of Wild-Type and Variant COMT Isoforms. Cancer Res September 15, 2001 61; 6716.
[lxxx] Shield AJ, Thomae BA, Eckloff BW, Wieben ED, Weinshilboum RM. Human catechol O-methyltransferase genetic variation: gene resequencing and functional characterization of variant allozymes. Molecular Psychiatry (2004) 9, 151–160.
[lxxxi] Åberg E, Fandiño-Losada A, Sjöholm LK, Forsell Y, Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. J Affect Disord. 2011 Mar;129(1-3):158-66.
[lxxxii] Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol-O-methyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: interaction with dietary energy intake. Am J Hypertens. 2011 Sep;24(9):1022-6.
[lxxxiii] Schalinske KL, Smazal AL. Homocysteine Imbalance: a Pathological Metabolic Marker. Adv Nutr November 2012 Adv Nutr vol. 3: 755-762, 2012.
[lxxxiv] Ziegler DA, Ashourian P, Wonderlick JS, Sarokhan AK, Prelec D, Scherzer CR, Corkin S. Motor impulsivity in Parkinson disease: associations with COMT and DRD2 polymorphisms. Scand J Psychol. 2014 Jun;55(3):278-86.
[lxxxv] Tammimäki A, Männistö PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012 Sep;22(9):673-91. doi: 10.1097/FPC.0b013e3283560c46. Review. PubMed PMID: 22722321.
[lxxxvi] NIH. AHCY. Retrieved March 15, 2016 from https://ghr.nlm.nih.gov/gene/AHCY
[lxxxvii] NIH. Hypermethioninemia. Retrieved March 15, 2016 from https://ghr.nlm.nih.gov/condition/hypermethioninemia
[lxxxviii] National Center for Biotechnology Information. AHCY. Retrieved March 15, 2016 from http://www.ncbi.nlm.nih.gov/gene/191
[lxxxix] Giusti B, Saracini C, Bolli P, Magi A, Sestini I, Sticchi E, Pratesi G, Pulli R, Pratesi C, Abbate R. Genetic analysis of 56 polymorphisms in 17 genes involved in methionine metabolism in patients with abdominal aortic aneurysm. J Med Genet. 2008 Nov;45(11):721-30. doi: 10.1136/jmg.2008.057851. Epub 2008 Jul 17. PubMed PMID: 18635682.
[xc] Boccia S, Brand A, Brand H, et al. The integration of genome-based information for common diseases into health policy and healthcare as a major challenge for Public Health Genomics: the example of the methylenetetrahydrofolate reductase gene in non-cancer diseases. Mutat Res. 2009 Jul 10;667(1-2):27-34. doi: 10.1016/j.mrfmmm.2008.10.003. Review. PMID: 19007797.