ENVIRONMENTAL MEDICINE
Volume 9, Number 2
Barbara A. Solomon M.D., M.A.
Abstract
The identification of food, mold, and other sensitivities in the therapy of environmentally ill patients can be cumbersome and lengthy. The use of the Alcat® Test, a Coulter Counter and computer integrated system which is able to measure white cell reactions to foods, molds and chemicals, was used to identify food and mold triggers in a group of 172 patients as seen in a primary care internal medical practice. Percentage improvement was averaged in various symptom categories. Percentage improvements in symptoms, as judged by the patients, and the speed and accuracy of the technique indicate that the Alcat® Test procedure is a beneficial addition to the armamentarium of the physical treating environmental illnesses. Environmental sensitivities, allergy, Alcat Test, Coulter Counter and computer integrated systems, white blood cell reaction, platelet reaction, double blind challenge, food allergy, food hypersensitivity, Serial Endpoint Titration, delayed reaction.
Introduction
This paper will demonstrate the potential benefits derived from the Alcat® Testing technique (1,2) in the practice of Environmental Illness. Fell et al (3) was able to design a diet based upon Alcat® Testing which helped in a number of clinical situations, including irritable bowel syndrome (IBS), allergic rhinitis, urticaria, eczema, and migraine. This author’s experience with Bryan’s cytotoxic testing (4) suggests that the above disorders and others are diet dependent. Early methods used to identify trigger foods included; cytotoxic testing; and oral food challenge. These methods are either cumbersome, as in the case of cytotoxic testing, where a technician’s opinions are “subjective”. Thus, the development of a reliable in-vitro test to check a patient’s blood to identify and eliminate food sensitivities was felt to be of substantive value. The Alcat® Test was found to be a quick and accurate method for diagnosing and managing diet related disorders.
Methods and Materials
The Alcat® Test system, as offered by AMTL in 1988, consisted of panels with ten foods, thirty foods, 50 foods, 100 foods and ten moulds. New patients whose symptoms were felt to be food and/or mold dependent, based upon answers given in an Allergy Data Base and Health History, were offered the appropriate food and mold panels. Food panels were selected to cover repeatedly eaten foods, and at the least cost to the patient. Patients included in this study were seen between January, 1989 and October 1990. Of these 192 cases, 7 were excluded because they declined skin testing and allergy injections, even though their mold Alcat® Test was positive and/or their health questionnaire was positive for mold, pollen or dust sensitivity. In ten patients, the food Alcat Test was positive for reactive foods, but the patients did not find that eliminating these foods helped their symptoms. These patients were also excluded. Some patients reported being worse during the first three days of elimination, but no patient reported being worse after the initial withdrawal period, lasting only three to five days.
After the appropriate food panel had been selected, equal 500 microliter aliquots of blood were pipetted into a test vial containing either allergen or allergen free buffer (to serve as a control). There were five control vials, in the 30, 50 and 100 food panels. Each sample was put through the analyzer. Using the Coulter method of particle sizing and counting, a size distribution curve depicting the total white blood cell and platelet population was made. At the end of a 90 minute incubation, 45 minutes at 36.6°C and 45 minutes at room temperature (21°-22° C), the interface computer program averaged the control curves, creating a master graph with the control samples and integrating a test graph for the blood reactions1,2 with each food or mold extract. The graph for each food was plotted against the master control graph. An algorithm determined the degree of shift or dislocation that occurred between each test graph and the master control. All cellular elements were present during incubation. A red-cell lysing solution was added to the mixture for 45 seconds before it was read by the Coulter Counter, lysing the red cells but not the white cells, enabling the measurement of the white cell and the platelet changes.
Distribution curves were plotted after the incubation with the food or mold extracts and plotted with the cell size on the abscissa and the cell number on the ordinate, then compared graphically to the control curve. Each food reaction was represented with two lines. They were superimposed graphically in the histogram, with one line representing the allergen free buffer and the other representing the response of the patient’s white cells and platelets.
Reading from left to right, the curve revealed the response of the platelets, followed by the lymphocytes and monocytes and on the right-end by the granulocytes. The region on the extreme left of the graph is the area where platelet agglutination is observed, if it occurs. The left peak, just to the right of the platelet area, is comprised of lymphocytes. Monocytes are to the right of the lymphocyte peak, and the granulocyte peak is noted on the far right side of the graph.
Earlier studies by Sandberg (5) indicated that changes in cell size were rarely greater than 0 to 9 percent in healthy subjects who did not have adverse or other reactions to foods. Changes of 10% to 12% are considered possible causes of adverse reactions to foods and those greater than 12% are considered probable causes of adverse reactions. Confirmation of sensitivity depends on relief of symptoms with food avoidance and the return of symptoms with reintroduction of the food in question. Platelet and white cell changes, as noted by Pasula (6) , were included in the designation of a food as reactive.
The results of the Alcat® Tests were discussed with each patient. The patient was instructed to avoid all foods that caused a 10% or greater reaction and/or a significant platelet reaction. Four-day rotation diets were devised, with appropriate food eliminations. The patients were seen at one to three month intervals. Three to six months later, Serial Endpoint Titration (SET), according to Rinkel (7,8), was carried out. A time separation of three to six months from the institution of an elimination diet to the institution of allergy skin testing and injection for inhalant allergies enabled the patient and author to evaluate improvements, or lack of improvements, separately. Immunotherapy was given weekly or biweekly, in addition to the four-day rotation and elimination diets. For those patients who were unable to follow an elimination diet, food extracts were devised using the Carlton Lee Technique (9) and administered by immunotherapy.
A total of 172 cases were reviewed for this study. One to two years after the diets were given an independent reviewer, unfamiliar with the subject matter, asked the patients to evaluate, on a scale of zero to ten the effectiveness of the elimination diets and/or allergy shots in alleviating their symptoms. The responses to food elimination and immunotherapy were assessed by the patients and were evaluated as two separate scores. The questions were kept simple so as not to confuse the patients in their responses. Improvement percentages were based on ratings given by the patient. A rating of 10 was a 100% improvement, clearing all symptoms. A rating of 5 was a 50% improvement. For the complaint of headaches or pain due to IBS, a rating of 10 was given if the headache, pain or diarrhea cleared completely. A 5 meant that headaches were 50% better in terms of frequency and severity. A score of zero was given when there was no diminution in symptoms.
The cases evaluated in this study included patients with the following clinical conditions: classic migraine; common migraine; sinus headaches; irritable bowel syndrome (IBS); gastroesphageal reflux (GERD); inflammatory arthritis; degenerative arthritis; asthma; recurrent sinusitis; tension fatigue syndrome due to allergy; depression and/or anxiety; obesity; eczema; recurrent vaginitis; recurrent cystitis; and allergic rhinitis. These conditions, in the author’s experience, were often triggered by foods. The number of patients in each category varied from a minimum of four recurrent urinary tract infections to a maximum of 108 with allergic rhinitis.
Results
The subcatagories of the elimination diet based upon the Alcat® Test and immunotherapy could be judged separately, since the immunotherapy was instituted three to six months after the institution of the elimination diet. The improvements associated on the elimination diets were usually evident within four to six weeks and were therefore complete before immunotherapy for the inhalant allergies had been started. The average number of symptoms per patient was 3.7, with extremes of one to nine.
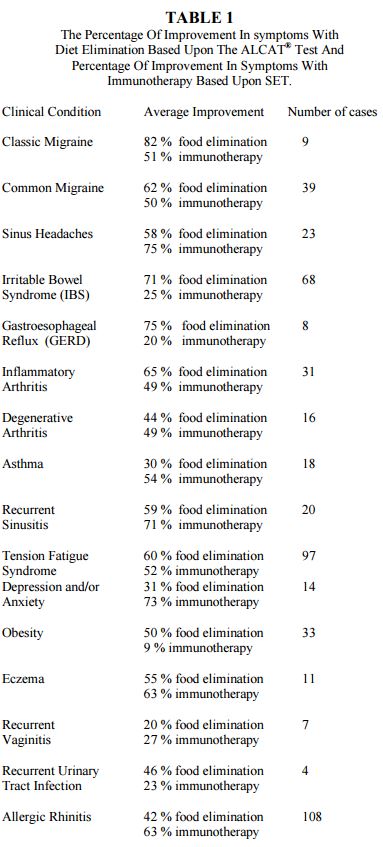
Table 1 lists the improvement percentages for the clinical conditions treated, broken down into the subcategories of food elimination based upon the Alcat® Testing, food extracts, where the reactive foods could not be avoided according to the Carlton Lee Technique (9) and immunotherapy based upon the Serial Endpoint Titration according to Rinkel. (7)
Discussion
The Alcat® Testing was most helpful in designing diets that were used for the treatment of patients with classic migraine (85%), common migraine (62%), sinus headaches (58%), gastroesophageal reflux (GERD) (75%), IBS (71%), inflammatory arthritis (65%), recurrent Sinusitis (59%), tension fatigue syndrome (60%), obesity (50%), and eczema (55%). It was less helpful in designing diets that were used for the management of the patients with asthma (30%), depression and/or anxiety (31%), recurrent vaginitis (20%), recurrent urinary tract infection (46%), degenerative arthritis (44%) and allergic rhinitis (42%). Many authors have indicated that food allergy is an important cause of migraine headaches,(10,17) GERD, (18,19) irritable bowel syndrome, (20-31) arthritis (32-42) tension fatigue syndrome, (43-51) asthma,52,56 obesity, (57-60) eczema,61,62 recurrent urinary tract syndrome, (63,64) and allergic rhinitis.(65-69)
Inhalant immunotherapy was the most useful for classic migraine (51%), common migraine (50%), sinus headaches (75%), asthma (54%), recurrent sinusitis (71%), tension fatigue syndrome (51%), depression and/or anxiety (71%), and allergic rhinitis (61%). It was intermediately helpful for inflammatory arthritis (49%), and degenerative arthritis (49%), but was less helpful for IBS (39%), GERD (20%), obesity (9%), recurrent vaginitis (27%), and recurrent urinary tract Infections (23%).
The food elimination diets, based on the Alcat® Test was a helpful in one case of Crohn’s disease (80% improvement) and two cases of children with Attention Deficit Disorder with the Hyperactivity (ADHD) (60% and 80% improvement). The first ADHD patient used food drops and a rotation diet. The second ADD case used an elimination diet. There were two cases of Parkinson’s disease with an 80% improvement on a restricted diet. One case of Alzheimer’s disease was felt to have improved 80% as a result of a restricted diet. Similar results have been obtained by others for ADHD (70,71) and Crohn’s disease. (31)
Alcat® Testing demonstrates several advantages over other forms of allergy testing. The Alcat® Test is specific to each patient. A food that is not on the standard IBS or migraine diet, but which bothers a patient, may be identified by Alcat® Test. The Alcat® Testing procedure correlate 79% with double blind challenge if positive, and 87.9% if negative.73 The positive platelet reactions noted on the Alcat® Test histograms (6) increase the positive correlation’s another 50%, bringing the positive correlation’s with double blind challenge to 87% to 92%.
The Alcat® Test is quick. Four-day fasting and oral challenge, done on an outpatient basis of in an ecology unit, can take as much as 25 days (at 3 to 4 foods a day). Alcat® Test can test 100 foods in four hours.
The Alcat® Test is simple. The system sizes and counts approximately 4000-8000 cells in six seconds. A trained cytotoxic technician cannot visually identify the subtle cell size changes, which are very accurately identified by the Alcat® Test system.
The Alcat® Test panels are predetermined. The foods and molds cannot be shifted or substituted within a panel. The Alcat® Test is a guide, which needs confirmation with avoidance and challenge, as it is not 100% accurate as compared to the standard set by an oral food challenge. Percentages of correlation for the Alcat® Test is 79.3% for positive reacting foods and higher (87%-92%), if platelet reacting foods are included. The percentage correlation for negative reacting foods is 87%. (73)
Crook (75) has noted that children with food allergies had more than one symptom. This is also true of adult patients. The average number of symptoms experienced by the patients in this series was 3.7, with a range from one to nine. Speer (76) noted that 86% of children who were sensitive to foods were also sensitive to inhalants, with molds and house dust being the most common offenders. In this study, 57% (93/162) of adult patients were also allergic to inhalants- dust, pollens, molds, and were treated with immunotherapy. Ninety-three of 162 patients with food allergies were thought to be inhalant sensitive. Ten of the original 172 cases were mold sensitive but not food sensitive.
The patients in this series usually came to the author as a last resort. Foods had not been thought previously to be triggering their symptoms. This may account for the relatively high percentage, which improved with food elimination.
Whereas these results are encouraging, further work needs to be done on the mechanism that causes the changes in cell size and number measured by the Alcat ® Test. The advantage of the Alcat® Test is that the operative mechanisms are multiple. Pasula and Puccio suggest that IgA, IgM and IgE are involved in the reactions.(77) Fell suggests that the toxic effect of foods may not always be immunological (74,78) and the effect may be cellular, for the molecules concerned may be to small to have any immunological effect. (74) It would be worthwhile to study these other mechanisms as this might shed light on the underlying pathologic mechanisms of adverse reactions to foods and molds. The Alcat® Test does not usually measure reactions, which are IgE mediated. According to M.J. Pasula, further research is underway to develop an Alcat® Test basophile degranulation test; this might help to measure a wider spectrum of allergic and/or pharmacologic reactions.
Summary
The advantages of speed and scope of the Alcat® Test system in testing foods, in terms of numbers of foods that can be tested at one time, more than compensates for the slight decrease in accuracy when compared to the standard of oral food challenge. In terms of objectivity and accuracy, it is superior to cytotoxic testing. Compared to standard IBS or standard migraine diets, the Alcat® Testing is much superior because it is specific to the patient. The Alcat® Tests for delayed hypersensitivity and other varieties of adverse reactions but usually not those which are IgE mediated reactions. Research is presently underway to develop an Alcat® Test basophil degranulation test, which could help the Alcat® Test technique measure a wider spectrum of allergic and/or pharmacologic reactions.
Literature
1. Pasula MJ. A Possible New Whole Blood Assay for Delayed Hypersensitivity Reactions. AMT Events Quarterly 1988:5:178-9.
2. Coulter Electronics. Coulter Counter Model ZM Product Reference Manual PN21234161, Healeah, Fl., Coulter Electronic Inc. 1984:2-15.
3. Fell PJ, Brostoff J, O’Donnell HO, et al. ALCAT®-“A New Test For Food Induced Problems in Medicine”. Annual Meeting of the American Academy of Otolaryngic Allergy, Oct. 1, 1988, Washington D.C. AAOA News 1988:6(2):29.
4. Bryan W, Bryan M. The Application of an In Vitro Cytoxic Reaction to Clinical Diagnosis of Food Allergy. Laryngoscope 1960:70:810-924.
5. Sandberg DH, Pasula MJ. A Comparison of the ALCAT® Test for Food Reactions Amongst Two Population Sub Groups. 45 th Annual Congress of the American College of Allergy and Immunology. Nov. 12-16, Los Angeles, Calif. Syllabus 1988:223.
6. Pasula M. Influence of Food Antigens on Volumes of Circulating White Blood Cells and Platelet Aggregation. Fourth International Symposium On Immunological and Clinical Problems of Food Allergy. Milano, Italy, 11/5-9/89. Abstract Symposium Book, p19.
7. Rinkel HF. Inhalant Allergy, Part I: The Whealing Response of the Skin to Serial Dilution Testing. Part II: Factors of Modifying the Whealing Response of the Skin. Part III: Coseasonal Application of Serial Dilution Testing (Testing). Ann All 1949:7:625-650.
8. Rinkel HF. The Management of Clinical Allergy. Arch Otolaryng 1962:76:489-500.
9. Lee C, Williams RT, Binkely EL. Provocative Testing and Treatment. Archives Otolaryngology 1969:90:87-95.
10. Rowe A. Allergic Toxemia and Migraine Due to Food Allergy. Cal & West Med 1930:33:785-92.
11. Randolph TG. Musculoskeletal Allergy in Children. Int Arch All 1959:14:84-96.
12. Andresen A. Migraine: An Allergic Phenomenon. Am J Dig Dis & Nutr 1934/5:1:14-17.
13. Glaser J. Migraine in Pediatric Practice. Am J Dis Children 1954:88:92-98.
14. Grant E. Oral Contraceptives, Smoking and Fodd Allergy. Lancet 1978:2:581-2.
15. Grant E. Food Allergies and Migraine. Lancet 1979:1:966-9.
16. Wilson CWM. The Clinical reactures of Migraine as a Manifestation of Allergic Disease. Post Grad Med 1980:56:617-21.
17. Egger J, Carter CM, Wilson J, et al. Is Migraine Food Allergy? Lancet 1983:ii:865-869.
18. Nebel OT. Symptomatic Gastroesophageal Reflux: Incidence and Precipitating Factors. Dig Dis 1976:21:953-6
19. Price SF. Food Sensitivity in Reflux Esophagitis. Gastroent 1978:75:240-3.
20. Vaughan WT. The Allergic Factor in Mucous Colitis. South Med J 1928:21:894-9.
21. Duke WW. Allergy As A Cause of Gastro-intestinal Disorder. Southern Med J 1931:24:363-7.
22. Rowe AH. Gastrointestinal Allergy. Jama 1931:97:1440-45.
23. Rowe AH. Food Allergy in the Differential Diagnosis of Abdominal Symptoms. Am J Med Sci 1932:183:529-37.
24. Friedenwald J, Morrison S. Food Allergy in its Relation to Gastrointestinal Disorders. Am J Dig Dis Nutr 1934/5:1:100-3
25. Smul JS. Clinical Evidence of Fifty So-Called Gastrointestinal Diseases Which Really Are Caused By Food Allergy With Discussion of Their Treatment. Am J Dig Dis Nutr 1935/6:2:178-184.
26. Gay LP. Muc. Colitis Complicated by Colonic Polyposis. Am J Dig Dis 1936:3:326-9.
27. Andersen AFR. Allergy of the Gastrintestinal Tract. Rev Gastroent 1951:18:779-90.
28. Jones V A, McLaughlan P, Shorthouse M, Morkman E, Hunter JD. Food Intolerance: A Major Factor in the Pathogenesis of Irritable Bowel Syndrome. Lancet 1982:ii: 1115-17.
29. Bently SJ, Pearson DJ, Rix KJ. Food Hypersensitivity in Irritable Bowel Syndrome. Lancet 1983:ii:295-7.
30. Gerrard JW. Food Intolerance. Lancet 1984:ii:413.
31. Jones VA, Hunter JO. Irritable Bowel Syndrome and Crohn’s Disease. In: Brostoff J, Challacombe SJ, Eds. Food Allergy and Intolerance. Bailliere Tindal. 1987:555-569.
32. Rinkel H. The Role of Food Allergy in Internel Medicine. Annals of Allergy 1944:2:115-24.
33. Randolph TG. Allergy as a Cause of Nuchal Myalgia and Associated Headache. Arch Otolar 1944:50:745-58.
34. Criep LH. Allergy of the joints. J Bone Joint Surgery 1946:28:276-9.
35. Zeller M. Rheumatoid Arthritis- Food Allergy as a Factor. Ann All 1949:7:200.
36. Randolph TG . Allergy as a Cause of Acute Tortocollis. Amer Prac Dig Treat 1950:1:1062.
37. Randolph TG. Allergic Myalgia. J Michigan State Medical Society 1951:50:487-494.
38. Kaufman W. The Overall Picture of Rheumatism and Arthritis Ann All 1952:10:47-52.
39. Randolph TG. Musculoskeletal Allergy in Children. Int Arch All 1959:14:84-96.
40. Hicklin JA, McEwen LM, Morgan JE. The Effect of Diet in Rheumatoid Arthritis. Clin All 1980:10:463-467.
41. Parke AL, Hughes GRV. Rheumatoid Arthritis and Food: A Case Study. Brit Med J 1981:282:2027-2029.
42. Wojtulewske JA. Joints and Connective Tissue. In: Brostoff J, Challacombe SJ, Eds. Food Allergy and Intolerance. Bailliere Tindall 1987:723-735.
43. Shannon WR.Neuropathic Manifestations in Infants and Children As a results of Anaphylactic Reactions to foods Contained in their Diet. Am J Dis Children 1922:24:89-94.
44. Randolph TG. Fatigue and Weakness of Allergic Origin (Allergic Toxemia) To Be Differentiated from Nervous Fatigue or Neurasthenia. Ann All 1946:3:418-30.
45. Randolph TG. Allergy as a Causative Factor of Fatigue, Irritability and Behavior Problems of Children. J Peds 1947:31:560-72.
46. Rowe A. Allergic Toxemia and Fatigue. Ann All 1950:8:72.
47. Speer F. The Allergic Tension – Fatigue Syndrome. Ped Clin North America 1954:1:1027-37.
48. Speer F. the Allergic Tension Fatigue in Children. Ann All 1954:12:168-71.
49. Kaufman W. The Comprehensive Management Of Patients Suffering from Food Induced Allergies. Int Arch All 1955:6:361-69.
50. Crook WG, Harrison W, Crawford S, Amerson BS. Systemic Manifestations due to Allergy, Pediatrics 1961:27:790.
51. Roth A. Detection of Food Allergy. Postgrad Med 1962:32:432-435.
52. Rinkel H. Food Allergy: Its Manifestations, Diagnosis and Treatment. JAMA 1928:91:1628.
53. Hill LW. Food Sensitivity in 100 Asthmatic Children. NEJM 1948:239:1039-1040.
54. Ogle KA, Bullock JO. Cjildren with Allergic Rhinitis and/or Bronchial Asthma. Ann All 1977:39:8-11.
55. Ogle KA, Bullock JD. Children with Allergic Rhinitis and/or Bronchial Asthma: A five Year Follow up. Ann All 1980:1980:44:273-278.
56. Wraithe DG. Asthma. In: Brostoff J, Challacombe SJ, Eds Food Allergy and Intolerance. Baillere, Tindall. 1987:486- 497.
57. Randolph TG. Masked Food Allergy as a Factor in the Development and Persistence of Obesity. Proceedings of the 20th Annual Meeting of Central Society for Clinical Research 1547-1549.
58. Randolph TG. The Descriptive Features of Food Addiction: Addictive Eating and Drinking. Quaterly J Alcoholism 1956:17:198-254.
59. Royal FF. Food Allergy – Addiction in a Bariatric Practice. Obesity/Bariatric Med 1978:7:2:56-58
60. McDowell M. Appetite Control: An Addiction-Like Component in Overeating and its Cure. Obesity/Bariatric Med 1980:9:5:138-142.
61. Atherton DJ, Sewell M, Soothill JF, Wills RS. A Double Blind Controlled Crossover Trial of an Anti-Avoidance Diet in Atopic Eczema. Lancet 1978:7:2:401-413.
62. Sampson HA. Role of Immediate Food Hyper-sensitivity in the Pathogenesis of Atopic Dermatitis. J All Clin Immunol 1983:71:473-80
63. Walter CK. Allergy as a cause of Genitourinary Symptoms: Clinical Consideration. Ann All 1958:16:158-9.
64. Sandberg D. Food Sensitivity: The Kidney and Bladder. In: Brostoff J, Challacombe SF, Eds. Food Allergy and intolerance. London, Bailliere, Tindall 1987:755-767.
65. Derlacke EL. Food Sensitization as a Cause of Perennial Nasal Allergy. Ann All 1955:13:682-89.
66. Gerrard JW. Familial Recurrent Rhinorrhea and Bronchitis Due to Cow’s milk. JAMA 1966:13:682-89.
67. Missal SC. Food Allergy in Ear, Nose and Throat Disease. Otolaryngologic Clinics of NA. 1971:4:3:479-91
68. Deamer WC. Pediatric Allergy: Some Impressions Gained Over a 37-Year Period. Peds 1971:48:930-8
69. Pelikan Z. Rhinitis and Secretory Otitis Media: A possible Role of Food Allergy and Intolerance. In: Brostoff J, Challacombe SJ, Eds. Food Allergy and Intolerance. London, Bailliere, Tindall. 1987:467-485.
70. Egger J, Carter CM, Graham PJ, Gumley D, Soothill JF. Controlled Trial of Oligoantigenic Treatment in the Hyperkinetic Syndrome. Lancet 1985:2:540-45.
71. Egger J, Stolla A, McEwen L. Controlled Trial of Hyposensitization in Children with Food-Induced Hyperkinetic Syndrome. Lancet 1992:339:1150-1153.
72. Egger J. The Hyperkinetic Syndrome. In: Brostoff J, Challacombe SJ, Eds. Food Allergy and Intolerance. London, Bailliere, Tindall. 1987:674-687.
73. Brostoff J, Fell PF, Pasula MJ. High Correlation of the ALCAT® Test Results with Double Blind Challenge (DBC) in Food Sensitivity. Presented at the 45th Annual Congress of the American College of Allergy and Immunology 11/12 – 16/88. Syllabus, p.213.
74. Fell PJ, Brostoff J, Pasula MJ. Pharmacoactive Compounds in Foods – The Effect on the ALCAT® Test in Healthy Volunters and patients Suffering from Migraine. AAOA News 9:2:29 75. Crook W. Food Allergy – The Great Masquerader. Ped Clinics North America 1975:22:227-38.
76. Speer F. Multiple Food Allergy. Ann All 1975:34:71-6.
77. Pasula MF, Puccio SG. Multiple Pathogenic Mechanisms in Food Sensitivity Reactions In Vitro. Fourth International Symposium on Immunological and Clinical Problems of Food Allergy. Milano, Italy. Nov. 5-9, 1989. Abstract Symposium Book, p.37.
78. Fell PJ. Cellular Responses to Food in Irritable Bowel Syndrome- An Investigation of the ALCAT® Test. J Nut Med 1991:2:143-149.